Aluminium‑ion Batteries
What is an Aluminium-ion battery and how does it work?
Energy‐storage technology is rapidly growing due to increasing demand from portable electronic devices and electrical vehicles. Currently, lithium-ion batteries (LIB) are one of the best energy storage system due to high energy density and long cycle life. However, limited resources of lithium (0.0065 wt % of the earth's crust), flammability, poisoning, and volatile electrolytes utilized in LIB system are against sustainable and green energy demands. This urges development in other battery system to be replaced with LIB. In other words, a battery system should have characteristics such as high safety, low price, high energy density, long cycle life, and high power density to be alternatives to LIB system. This means anode, cathode and electrolyte in a new battery system should possess characteristics as following:
- Cheap and abundant materials
- environmentally friendly, non‐flammable, and low toxicity
- materials with a high capacity and a reasonable operating voltage
- good reversibility of the anode/cathode reaction and an acceptable electrolyte with electrochemically stable window, which provides a long service life
- fast kinetics of the electrochemical reactions to reduce the polarization and the energy lost during charge/discharge.
Aluminum-ion batteries have been considered as promising candidate for post–lithium battery. In Al-ion battery, multivalent ions transfer of Al3+ results to a high theoretical capacity, once it is coupled to a suitable cathode material. The specific volumetric capacity of an Al anode is up to 8046 mAh cm−3, which is approximately four times higher than Li. In addition, Al is one of the most abundant elements on the earth (8 wt% of earth's crust), with low cost, compared to Li. Moreover, using Al as the anode lower the potential safety hazard due to more air stability compared to Li, which also benefits ease of handling in ambient environment. Also, the ionic liquid electrolyte used in Al batteries is non‐volatile and nonflammable, which gives a lower safety risk. Using graphitic carbon material as a cathode is ideal for intercalation with Al ions in a cathode sites, which has high electrical conductivity and low cost. Recently, AIB based on graphitic carbon materials has shown a significant progress.
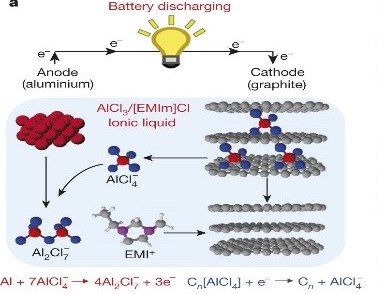
Figure: Schematic picture of Aluminum-graphite cell using ionic electrolyte during discharging.
Challenges / Possibilities
Various challenges have limited the AIB performance. One is the capacity of the graphitic material cathode, which has not achieved to a satisfactory value, due to rather ambiguous intercalation mechanism of [AlCl4]– towards the cathode. Thus, advances in the cathode material design and understanding of intercalation mechanism is necessary to enhance the cathode capacity in AIB. Moreover, finding a suitable electrolyte for aluminum-ion battery is ongoing. Currently, EMIMCI is an ionic liquid solvent, which is commonly used in Al-ion battery. For environmental reasons aqueous electrolyte system are preferred, however these electrolytes cause corrosion problems as well. Moreover, scalability is a technological challenge. With developing a new concept based upon salt-carrying paper-based separator, coated graphene/graphite electrodes, and aluminum foils, a low-cost alternative can be achieved.
Our research
Rechargeable Aluminum-ion Battery with graphene composite cathode